- 28 April 2021
- Vijay
- 0
ISO 13485 Certification Consulting
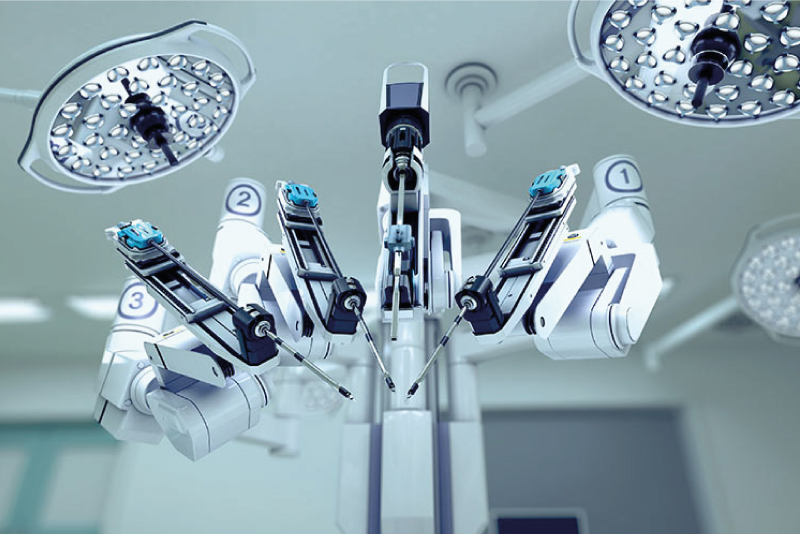
ISO 13485 is the medical device industry’s most widely used international standard for quality management. Issued by the International Organization for Standardization (ISO), the ISO 13485 standard is an effective solution to meet the comprehensive requirements for a Quality Management System in the medical device industry.
Adopting ISO 13485 provides a practical foundation for manufacturers to address the EU Medical Device Directive, the EU Medical Device Regulation, and other regulations, as well as demonstrating a commitment to the safety and quality of medical devices.
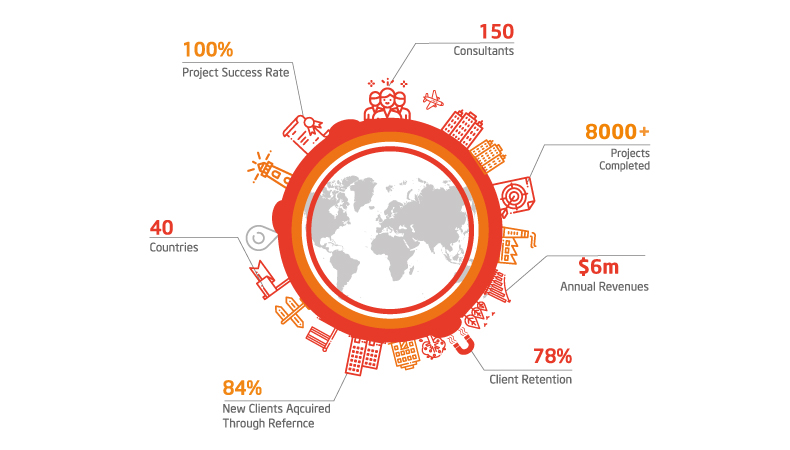
Why Work With Quality Catalyst?
100
%
Project Success Rate
150
Consultants Projects Completed
3000
+
New Clients Acquired Through ReferenceThe benefits of implementing ISO 13485 Certification include:
- Contracting with Larger Organizations
- Demonstrate a commitment to excellence.
- Increase your market’s potential size
- Assist Staff in Obtaining Relevant Information
- Consolidate and expand company knowledge